ocrevus start up form
It must be completed by the provider. MEDICARE FORM For Medicare Advantage Part B.

Ocrevus Ocrelizumab Ms Infusion Experience
OCREVUS START FORM Century Specialty Script Fax Referral To.

. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS. Your healthcare provider can enroll you in this registry by calling 1-833-872-4370 or visiting. Swelling of the throat.
Access the OCREVUS Start Form and learn more. The form includes patient insurance and prescription information. Ad Check Out The Efficacy Safety Of TYSABRI.
Deciding If TYSABRI May Be An Option. Ad Discover Why More People Are Choosing To Start With Or Switch To OCREVUS. _____ Current Patient New Patient Need by date.
Ocrevus ocrelizumab Medication Precertification Request Page 1 of 2 Phone. Date of birth. Ad View 5 Years of Safety Data - 2 Years Controlled 3 Years Open Label Extension.
New patient Current patient Patients first name Last name Middle initial Male Female Last 4 digits of SSN Date of. Ocrevus Order FormPlease fax form to. These infusion reactions can happen for up to 24 hours after your infusion.
By completing this form you are requesting services on behalf of your patient which may include. It is important that. OCREVUS Start Form Ready to get started.
Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV to a final concentration of 12mgmL Every 6 months infuse 600mg in 500mL of 09 NS. Prescribers first name. To use Quick Enroll for the OCREVUS Start Form select E-Submit.
OCREVUS is a prescription medicine used to treat. PRIOR AUTHORIZATION FORM Ocrevus - CommercialMedicaid Unless otherwise indicated below authorization quantities are limited to the manufacturer recommended dosage. Review TYSABRIs Well-Undesrtood Safety Profile.
M F Patient Address. Ad Discover Why More People Are Choosing To Start With Or Switch To OCREVUS. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions.
If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy. Send all pages of the completed form to us by mail fax or email as noted below. Learn More About OCREVUS Resources And How Patient Navigators Can Help.
Ocrevus ocrelizumab Fax completed form to 8883021028. Swelling of the throat. Is this a new start or continuation of therapy.
Physicians are encouraged to. If Century Specialty Script is the patients choice please Call-In Fax or Mail prescriptions to. Ad View 5 Years of Safety Data - 2 Years Controlled 3 Years Open Label Extension.
Start at 30mlhr increasing by 30mlhr every 30 minutes to a. 300 mg250 ml NS IV on day 1. Benefits investigation Benefits reverification approximately 6.
As email is not a secure medium any person with concerns about their prior authorization formmedical. Ocrevus ocrelizumab Medication Precertification Request Aetna Precertification Notification Phone. Deciding If TYSABRI May Be An Option.
If you would like to receive any non-live inactivated vaccines. Learn More About OCREVUS Resources And How Patient Navigators Can Help. Prior Authorization Form for.
Review TYSABRIs Well-Undesrtood Safety Profile. The purpose of this registry is to collect information about your health and your babys health. See Website For Safety Boxed Warning PI.
Prescription Enrollment Form. OCREVUS START FORM Century Specialty Script Fax Referral To. Ad Check Out The Efficacy Safety Of TYSABRI.
Patients first name. Each vial contains 300 mg10 mL of OCREVUS for intravenous infusion. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary.
There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy. 405- 726-9849 Patient Information Patient Name. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and.
OCREVUS START FORM Y Medical Associates Fax Referral To. Content updated daily for ocrevus start form. 1-888-267-3277 For Medicare Advantage Part B.
See Website For Safety Boxed Warning PI.
Ectrims Roche Touts 8 Year Ocrevus Data To Defend Multiple Sclerosis Leadership Fierce Pharma

Ocrevus For Primary Progressive Ms What You Need To Know Ms Trust
Zeposia Ozanimod For The Treatment Of Multiple Sclerosis

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions

Ocrevus Ocrelizumab Ms Infusion Experience

Editable Body Contouring Consent Forms Bundle Body Sculpting Etsy Body Sculpting Body Contouring Nail Salon Design

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions

Look Out Biogen And Novartis Roche S Ocrevus Is Coming For Your Ms Sales Big Time Analyst Predicts Fierce Pharma

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Side Effects Cost Uses And More

Ocrevus Side Effects Cost Uses And More

Ocrevus Ocrelizumab Ms Infusion Experience
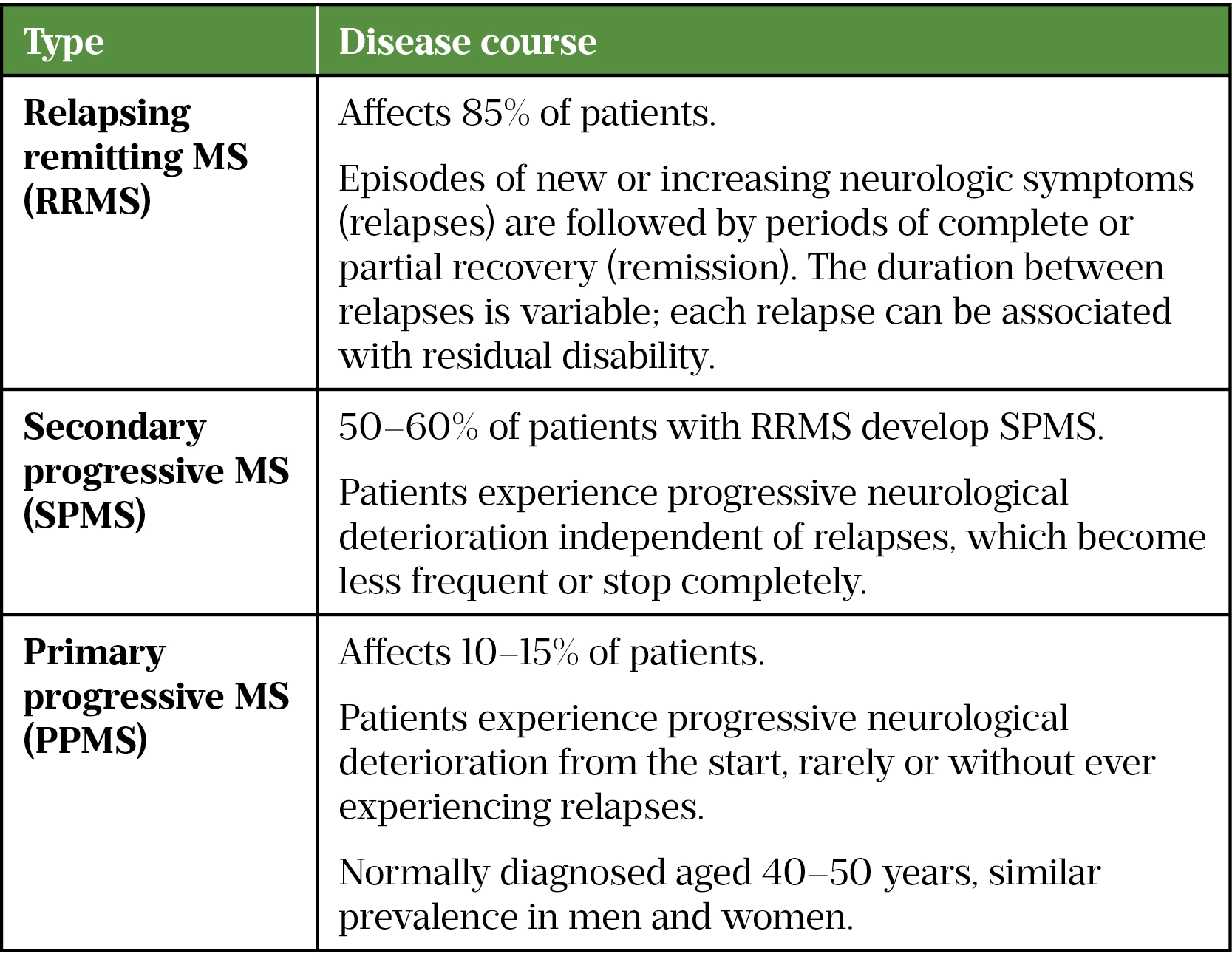
Multiple Sclerosis Disease Modifying Therapies The Pharmaceutical Journal

Multiple Sclerosis Disease Modifying Therapies The Pharmaceutical Journal


